Conditions Around Metabolically Active Tissues Do What to the Oxyhemoglobin Dissociation Curve?
The oxygen–hemoglobin dissociation curve, also chosen the oxyhemoglobin dissociation curve or oxygen dissociation curve (ODC), is a curve that plots the proportion of hemoglobin in its saturated (oxygen-laden) class on the vertical centrality against the prevailing oxygen tension on the horizontal axis. This bend is an important tool for agreement how our blood carries and releases oxygen. Specifically, the oxyhemoglobin dissociation bend relates oxygen saturation (SouthwardO 2) and partial pressure of oxygen in the blood (PO 2), and is adamant by what is chosen "hemoglobin analogousness for oxygen"; that is, how readily hemoglobin acquires and releases oxygen molecules into the fluid that surrounds it.
Construction of oxyhemoglobin
Background [edit]
Hemoglobin (Hb) is the main vehicle for transporting oxygen in the blood. Each hemoglobin molecule has the capacity to carry four oxygen molecules. These molecules of oxygen bind to the iron of the heme prosthetic group.[one]
When hemoglobin has no bound oxygen, nor bound carbon dioxide, information technology has the unbound conformation (shape). The binding of the first oxygen molecule induces alter in the shape of the hemoglobin that increases its power to demark to the other three oxygen molecules.
In the presence of dissolved carbon dioxide, the pH of the blood changes; this causes another modify in the shape of hemoglobin, which increases its ability to bind carbon dioxide and decreases its power to bind oxygen. With the loss of the first oxygen molecule, and the binding of the first carbon dioxide molecule, yet some other change in shape occurs, which farther decreases the ability to bind oxygen, and increases the ability to bind carbon dioxide. The oxygen bound to the hemoglobin is released into the blood's plasma and absorbed into the tissues, and the carbon dioxide in the tissues is bound to the hemoglobin.
In the lungs the reverse of this process takes identify. With the loss of the first carbon dioxide molecule the shape again changes and makes information technology easier to release the other three carbon dioxides.
Oxygen is also carried dissolved in the blood'due south plasma, but to a much bottom degree. Hemoglobin is contained in blood-red blood cells. Hemoglobin releases the bound oxygen when carbonic acid is present, as information technology is in the tissues. In the capillaries, where carbon dioxide is produced, oxygen jump to the hemoglobin is released into the blood's plasma and captivated into the tissues.
How much of that capacity is filled by oxygen at any time is chosen the oxygen saturation. Expressed equally a pct, the oxygen saturation is the ratio of the corporeality of oxygen bound to the hemoglobin, to the oxygen-carrying capacity of the hemoglobin. The oxygen-carrying capacity of hemoglobin is determined by the type of hemoglobin present in the blood. The amount of oxygen bound to the hemoglobin at whatsoever time is related, in big role, to the partial pressure level of oxygen to which the hemoglobin is exposed. In the lungs, at the alveolar–capillary interface, the partial pressure of oxygen is typically high, and therefore the oxygen binds readily to hemoglobin that is present. As the blood circulates to other body tissue in which the partial pressure of oxygen is less, the hemoglobin releases the oxygen into the tissue considering the hemoglobin cannot maintain its full bound capacity of oxygen in the presence of lower oxygen fractional pressures.
Sigmoid shape [edit]

Hemoglobin saturation curve
The curve is usually best described by a sigmoid plot, using a formula of the kind:
A hemoglobin molecule tin can bind up to four oxygen molecules in a reversible method.
The shape of the curve results from the interaction of bound oxygen molecules with incoming molecules. The binding of the first molecule is difficult. Still, this facilitates the binding of the second, tertiary and quaternary, this is due to the induced conformational change in the construction of the hemoglobin molecule induced by the bounden of an oxygen molecule.
In its about simple form, the oxyhemoglobin dissociation curve describes the relation betwixt the partial pressure of oxygen (x axis) and the oxygen saturation (y axis). Hemoglobin's affinity for oxygen increases as successive molecules of oxygen bind. More molecules bind equally the oxygen fractional force per unit area increases until the maximum amount that tin can be bound is reached. As this limit is approached, very piffling additional bounden occurs and the curve levels out every bit the hemoglobin becomes saturated with oxygen. Hence the curve has a sigmoidal or S-shape. At pressures above most 60 mmHg, the standard dissociation curve is relatively apartment, which means that the oxygen content of the blood does not change significantly fifty-fifty with big increases in the oxygen fractional pressure level. To get more oxygen to the tissue would require blood transfusions to increase the hemoglobin count (and hence the oxygen-carrying capacity), or supplemental oxygen that would increase the oxygen dissolved in plasma. Although binding of oxygen to hemoglobin continues to some extent for pressures about fifty mmHg, equally oxygen partial pressures decrease in this steep surface area of the curve, the oxygen is unloaded to peripheral tissue readily as the hemoglobin'due south affinity diminishes. The partial force per unit area of oxygen in the blood at which the hemoglobin is 50% saturated, typically virtually 26.six mmHg (three.v kPa) for a salubrious person, is known as the P50. The P50 is a conventional measure of hemoglobin affinity for oxygen. In the presence of illness or other conditions that modify the hemoglobin oxygen affinity and, consequently, shift the curve to the correct or left, the P50 changes accordingly. An increased P50 indicates a rightward shift of the standard curve, which means that a larger partial force per unit area is necessary to maintain a 50% oxygen saturation. This indicates a decreased affinity. Conversely, a lower Pfifty indicates a leftward shift and a higher affinity.
The 'plateau' portion of the oxyhemoglobin dissociation curve is the range that exists at the pulmonary capillaries (minimal reduction of oxygen transported until the p(O2) falls fifty mmHg).
The 'steep' portion of the oxyhemoglobin dissociation bend is the range that exists at the systemic capillaries (a small drib in systemic capillary p(O2) can result in the release of large amounts of oxygen for the metabolically active cells).
To see the relative affinities of each successive oxygen as you lot remove/add together oxygen from/to the hemoglobin from the curve compare the relative increase/decrease in p(Oii) needed for the corresponding increase/decrease in s(Oii). 69
Factors that touch the standard dissociation curve [edit]
The strength with which oxygen binds to hemoglobin is affected by several factors. These factors shift or reshape the oxyhemoglobin dissociation curve. A rightward shift indicates that the hemoglobin under study has a decreased analogousness for oxygen. This makes information technology more difficult for hemoglobin to bind to oxygen (requiring a higher partial pressure of oxygen to attain the same oxygen saturation), just it makes it easier for the hemoglobin to release oxygen jump to information technology. The outcome of this rightward shift of the curve increases the fractional force per unit area of oxygen in the tissues when it is most needed, such as during practice, or hemorrhagic shock. In contrast, the curve is shifted to the left past the contrary of these conditions. This leftward shift indicates that the hemoglobin under written report has an increased affinity for oxygen so that hemoglobin binds oxygen more easily, only unloads it more reluctantly. Left shift of the curve is a sign of hemoglobin's increased affinity for oxygen (eastward.g. at the lungs). Similarly, right shift shows decreased affinity, as would appear with an increase in either body temperature, hydrogen ions, 2,iii-bisphosphoglycerate (2,three-BPG) concentration or carbon dioxide concentration.
| Control factors | Change | Shift of curve |
|---|---|---|
| Temperature | ↑ | → |
| ↓ | ← | |
| 2,iii-BPG | ↑ | → |
| ↓ | ← | |
| pCO2 | ↑ | → |
| ↓ | ← | |
| Acidity [H+] | ↑ | → |
| ↓ | ← |
Annotation:
- Left shift: higher Otwo affinity
- Right shift: lower O2 analogousness
- fetal hemoglobin has college O2 analogousness than adult hemoglobin; primarily due to much-reduced analogousness to two,3-bisphosphoglycerate .
The causes of shift to right can be remembered using the mnemonic, "Cadet, face Correct!" for CO2, Acid, 2,iii-DPG,[Notation i] Exercise and Temperature.[2] Factors that move the oxygen dissociation curve to the right are those physiological states where tissues need more oxygen. For case, during practice, muscles have a college metabolic rate, and consequently need more oxygen, produce more carbon dioxide and lactic acid, and their temperature rises.
pH [edit]
A subtract in pH (increase in H+ ion concentration) shifts the standard curve to the right, while an increase shifts it to the left. This occurs considering at greater H+ ion concentration, various amino acid residues, such equally Histidine 146 exist predominantly in their protonated form allowing them to form ion pairs that stabilize deoxyhemoglobin in the T state.[3] The T state has a lower affinity for oxygen than the R state, so with increased acidity, the hemoglobin binds less Oii for a given PO2 (and more H+). This is known as the Bohr issue.[4] A reduction in the total binding capacity of hemoglobin to oxygen (i.e. shifting the curve down, not just to the right) due to reduced pH is called the root effect. This is seen in bony fish. The binding affinity of hemoglobin to O2 is greatest nether a relatively high pH.
Carbon dioxide [edit]
Carbon dioxide affects the curve in two ways. First, CO2 accumulation causes carbamino compounds to be generated through chemical interactions, which demark to hemoglobin forming carbaminohemoglobin . COtwo is considered an Allosteric regulation as the inhibition happens not at the bounden site of hemoglobin.[5] Second, it influences intracellular pH due to formation of bicarbonate ion. Formation of carbaminohemoglobin stabilizes T state hemoglobin by formation of ion pairs.[3] Just near 5–10% of the full CO2 content of claret is transported as carbamino compounds, whereas (80–90%) is transported as bicarbonate ions and a modest amount is dissolved in the plasma. The formation of a bicarbonate ion will release a proton into the plasma, decreasing pH (increased acidity), which also shifts the bend to the right every bit discussed above; low COtwo levels in the claret stream results in a loftier pH, and thus provides more optimal binding atmospheric condition for hemoglobin and O2. This is a physiologically favored machinery, since hemoglobin will drop off more than oxygen as the concentration of carbon dioxide increases dramatically where tissue respiration is happening rapidly and oxygen is in need.[6] [7]
2,3-BPG [edit]
ii,iii-Bisphosphoglycerate or 2,three-BPG (formerly named ii,3-diphosphoglycerate or 2,3-DPG - reference?) is an organophosphate formed in red blood cells during glycolysis and is the cohabit base of 2,3-bisphosphoglyceric acid. The production of two,3-BPG is likely an important adaptive machinery, because the production increases for several conditions in the presence of diminished peripheral tissue O2 availability, such as hypoxemia, chronic lung disease, anemia, and congestive heart failure, among others. High levels of 2,3-BPG shift the curve to the right (every bit in childhood), while depression levels of 2,3-BPG crusade a leftward shift, seen in states such equally septic shock, and hypophosphataemia.[4] In the absence of two,three-BPG, hemoglobin'southward affinity for oxygen increases. two,three-BPG acts as a heteroallosteric effector of hemoglobin, lowering hemoglobin's affinity for oxygen by binding preferentially to deoxyhemoglobin. An increased concentration of BPG in scarlet blood cells favours formation of the T (taut or tense), low-affinity country of hemoglobin so the oxygen-binding curve will shift to the right.
Temperature [edit]
Increment in temperature shifts the ODC to the right. If temperature is increased keeping the the same, and so the oxygen saturation decreases because the bond between fe in the and gets denatured. Similarly, with increase in temperature, partial pressure of oxygen besides increases. So, one volition have a lesser hemoglobin saturation percentage for the same or a higher partial pressure of oxygen. Thus, whatsoever point in the curve will shift rightwards (due to increased partial force per unit area of oxygen) and downwardly (due to weakened bail). Hence, the rightward shift of the bend.[eight]
Carbon monoxide [edit]
Hemoglobin binds with carbon monoxide 210 times more readily than with oxygen.[iv] Because of this higher affinity of hemoglobin for carbon monoxide than for oxygen, carbon monoxide is a highly successful competitor that will readapt oxygen even at minuscule partial pressures. The reaction HbO2 + CO → HbCO + O2 almost irreversibly displaces the oxygen molecules forming carboxyhemoglobin; the binding of the carbon monoxide to the atomic number 26 centre of hemoglobin is much stronger than that of oxygen, and the binding site remains blocked for the remainder of the life cycle of that affected ruby blood cell.[ix] With an increased level of carbon monoxide, a person can suffer from severe tissue hypoxia while maintaining a normal pOtwo because carboxyhemoglobin does not comport oxygen to the tissues.
Effects of methemoglobinaemia [edit]
Methemoglobinaemia is a form of abnormal hemoglobin where the iron heart has been oxidised from the ferrous +2 oxidation state (the normal form) to the ferric +three state. This causes a leftward shift in the oxygen hemoglobin dissociation curve, every bit any remainder heme with oxygenated ferrous atomic number 26 (+2 state) is unable to unload its bound oxygen into tissues (because 3+ iron impairs hemoglobin's cooperativity), thereby increasing its affinity with oxygen. Yet, methemoglobin has increased affinity for cyanide, and is therefore useful in the treatment of cyanide poisoning. In cases of accidental ingestion, administration of a nitrite (such as amyl nitrite) can be used to deliberately oxidise hemoglobin and raise methemoglobin levels, restoring the functioning of cytochrome oxidase. The nitrite also acts equally a vasodilator, promoting the cellular supply of oxygen, and the add-on of an iron salt provides for competitive binding of the gratuitous cyanide as the biochemically inert hexacyanoferrate(Iii) ion, [Iron(CN)6]3−. An alternative approach involves administering thiosulfate, thereby converting cyanide to thiocyanate, SCN−, which is excreted via the kidneys. Methemoglobin is besides formed in small quantities when the dissociation of oxyhemoglobin results in the germination of methemoglobin and superoxide, O2 −, instead of the usual products. Superoxide is a gratis radical and causes biochemical damage, but is neutralised by the activity of the enzyme superoxide dismutase.
Effects of ITPP [edit]
Myo-inositol trispyrophosphate (ITPP), also known as OXY111A, is an inositol phosphate that causes a rightward shift in the oxygen hemoglobin dissociation curve through allosteric modulation of hemoglobin within red claret cells. It is an experimental drug intended to reduce tissue hypoxia. The effects appear to final roughly as long as the affected red blood cells remain in circulation.
Fetal hemoglobin [edit]
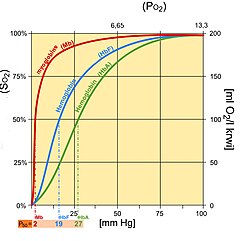
Fetal hemoglobin saturation curve
Fetal hemoglobin (HbF) is structurally dissimilar from normal adult hemoglobin (HbA), giving HbF a higher affinity for oxygen than HbA. HbF is composed of two alpha and two gamma bondage whereas HbA is composed of two blastoff and two beta chains. The fetal dissociation bend is shifted to the left relative to the curve for the normal adult because of these structural differences.
Typically, fetal arterial oxygen pressures are lower than adult arterial oxygen pressures. Hence higher affinity to bind oxygen is required at lower levels of fractional pressure level in the fetus to allow diffusion of oxygen across the placenta. At the placenta, there is a higher concentration of ii,3-BPG formed, and two,3-BPG binds readily to beta chains rather than to blastoff bondage. As a result, 2,3-BPG binds more than strongly to adult hemoglobin, causing HbA to release more oxygen for uptake by the fetus, whose HbF is unaffected by the ii,iii-BPG.[10] HbF then delivers that bound oxygen to tissues that have fifty-fifty lower partial pressures where it can be released.
See also [edit]
- Automatic analyzer
- Bohr upshot
Notes [edit]
- ^ two,3-DPG is an abbreviation of 2,three- D i P hospho G lyceric acrid, an obsolete name for 2,3-BPG
References [edit]
- ^ Ahern, Kevin; Rajagopal, Indira; Tan, Taralyn (2017). Biochemistry Complimentary For All (PDF) (one.two ed.). NC: Creative Eatables.
- ^ "Medical mnemonics". LifeHugger. Retrieved 2009-12-19 .
- ^ a b Lehninger. Principles of Biochemistry (6th ed.). p. 169.
- ^ a b c Jacquez, John (1979). Respiratory Physiology. McGraw-Colina. pp. 156–175.
- ^ Ahern, Kevin; Rajagopal, Indira; Tan, Taralyn (5 August 2017). Biochemistry Free For All (1.ii ed.). NC-Artistic Commons. p. 370.
- ^ Ahern, Kevin; Rajagopal, Indira; Tan, Taralyn (5 Baronial 2017). Biochemistry Gratuitous For All (i.2 ed.). NC-Artistic Commons. p. 134.
- ^ Donna, Larson (2017). Clinical Chemistry: Fundamentals And Laboratory techniques. St. Louis, Missouri: Elsevier. p. 226. ISBN978-1-4557-4214-ane.
- ^ Schmidt-Nielsen (1997). Creature Physiology: Adaptation and Environs. Cambridge University Press. ISBN0521570980.
- ^ Kotz, John (Baronial 2012). Chemical science and Chemical Reactivity (8th ed.). Cengage Learning. p. 1032. ISBN978-1133420071 . Retrieved 2015-07-01 .
- ^ Lippincott'due south Illustrated Review: Biochemistry 4th edition. North America: Lippincott, Williams, and Wilkins. 2007. pp. 24–35. ISBN978-0-7817-6960-0.
External links [edit]
- Nosek, Thomas M. "Department four/4ch5/s4ch5_18". Essentials of Man Physiology. Archived from the original on 2016-03-24.
- The Interactive Oxyhemoglobin Dissociation Bend
- Simulation of the parameters CO2, pH and temperature on the oxygen–hemoglobin dissociation curve (left or right shift)
Source: https://en.wikipedia.org/wiki/Oxygen%E2%80%93hemoglobin_dissociation_curve

![Oxygen concentration {\displaystyle {\ce {[O2]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8f8f06466530fdc7eb5967cec6cbfed6a59582aa)



0 Response to "Conditions Around Metabolically Active Tissues Do What to the Oxyhemoglobin Dissociation Curve?"
Postar um comentário